- Product Details
Keywords
- High quality Artesunate
- competitive price Artesunate
- Artesunate supplier
Quick Details
- ProName: High quality competitive price Artesun...
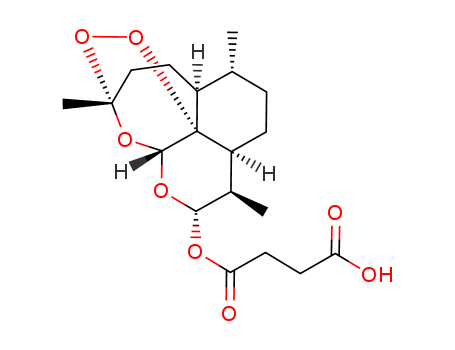
- CasNo: 88495-63-0
- Molecular Formula: C19H28O8
- Appearance: White color cream crystalline powder
- Application: Pharm
- PackAge: 1kg/Tin, 5kg/Tin, 20kg/Drum, 25kg/Drum...
- ProductionCapacity: Metric Ton/Day
- Purity: 99%
- Storage: Store in dry places and keep away from...
- LimitNum: 1 Kilogram
Superiority
1.USP/EP/BP/FCC/CP 2.GMP/DMF/KOSHER/HALAL/HACCP 3.10 Years Experience 4.Delivery within 5 days 5.OEM ability …
Details
1.USP/EP/BP/FCC/CP
2.GMP/DMF/KOSHER/HALAL/HACCP
3.10 Years Experience
4.Delivery within 5 days
5.OEM ability
Artesunate (INN) is part of the artemisinin group of drugs that treat malaria. It is a semi-synthetic derivative of artemisinin that is water-soluble and may therefore be given by injection. It is sometimes abbreviated AS.
Artesunate is used primarily as treatment for malaria; it has also been shown to be >90% efficacious at reducing egg production in Schistosoma haematobium infection.According to WHO intravenous AS is the drug of choice for severe malaria both in children and adults where there is low transmission. In regions where transmission is high, randomized trials among Asian adults and African children strongly advocates the use of artesunate over quinine as the treatment of choice for falciparum malaria.
2.Properties of Artesunate
(1)ACD/LogP: 3.291 (2)# of Rule of 5 Violations: 0 (3)ACD/LogD (pH 5.5): 2.04 (4)ACD/LogD (pH 7.4): 0.26 (5)ACD/BCF (pH 5.5): 10.55 (6)ACD/BCF (pH 7.4): 1.00 (7)ACD/KOC (pH 5.5): 83.06 (8)ACD/KOC (pH 7.4): 1.37 (9)#H bond acceptors: 8 (10)#H bond donors: 1 (11)#Freely Rotating Bonds: 5 (12)Polar Surface Area: 100.52 ?2 (13)Index of Refraction: 1.544 (14)Molar Refractivity: 92.21 cm3 (15)Molar Volume: 292.249 cm3 (16)Polarizability: 36.555 10-24cm3 (17)Surface Tension: 51.4119987487793 dyne/cm (18)Density: 1.315 g/cm3 (19)Flash Point: 175.638 °C (20)Enthalpy of Vaporization: 85.066 kJ/mol (21)Boiling Point: 507.123 °C at 760 mmHg (22)Vapour Pressure: 0 mmHg at 25°C
3.Structure Descriptors of Artesunate
SMILES:
O=C(O)CCC(=O)O[C@@H]3O[C@@H]4O[C@@]2(OO[C@]41[C@@H](CC[C@@H](C)[C@@H]1CC2)[C@H]3C)C
Std. InChI:
InChI=1S/C19H28O8/c1-10-4-5-13-11(2)16(23-15(22)7-6-14(20)21)24-17-19(13)12(10)8-9-18(3,25-17)26-27-19/h10-13,16-17H,4-9H2,1-3H3,(H,20,21)/t10-,11-,12+,13+,16-,17-,18-,19-/m1/s1
Std. InChIKey:
FIHJKUPKCHIPAT-AHIGJZGOSA-N
4.Synthesis of Artesunate
Artesunate is prepared from dihydroartemisinin (DHA) by reacting it with succinic acid anhydride in basic medium. Pyridine as base/solvent, sodium bicarbonate in chloroform and catalyst DMAP (N,N-dimethylaminopyridine) and triethylamine in 1,2-dichloroethane have been used, with yields of up to 100%. A large scale process involves treatment of DHA in dichloromethane with a mixture of pyridine, a catalytic amount of DMAP and succinic anhydride. The dichloromethane mixture is stirred for 6–9 h to get artesunate in quantitative yield. The product is further re-crystallized from dichloromethane. alpha-Artesunate is exclusively formed (m.p 135–137?C).
5.Application of Artesunate
Artesunate is recommended by the World Health Organization (WHO) in preference to quinidine for the treatment of severe malaria and has been used worldwide for many years. Artesunate is in the class of medications known as artemisinins, which are derivatives from the "quinghaosu" or sweet wormwood plant (Artemisia annua).
On June 21, 2007, the Food and Drug Administration (FDA) approved investigational new drug (IND) protocol # 76,725, entitled Intravenous Artesunate for Treatment of Severe Malaria in the United States. This IND makes a new class of antimalarial medication, artemisinins, available in the United States for the first time.
The Walter Reed Army Institute of Research (WRAIR) has been conducting studies in several countries using artesunate, and has agreed to provide a supply of this medication to CDC.
6. Package information
1kg/Tin, 5kg/Tin, 20kg/Drum, 25kg/Drum, 50kg/Drum, 200kg/Drum, 1MT/Drum
.jpg)
7. Delivery information
Delivery within 5 days
.jpg)